6.4 electronic structure of atoms (electron configurations) – chemistry Periodic table numbers of neutrons protons and electrons Atoms and elements
Atoms and Elements - Científicos Matemáticos
Atoms protons electrons neutrons many elements least number same atom most will
Neon protons neutrons electrons properties configuration table periodic electron atomic affinity number electronegativity mass ionization density gas two elements
Notation nuclear protons neutrons electrons nucleus periodic electron calcium magnesium nuclei xenon proton nuclide identifying chemistry antimony chemical atoms reactions7 pics periodic table of elements list with protons neutrons and Neutrons protons electrons number many nuclide atoms notation isotopes ions periodic atomic table proton mass neutron electron element numbers sameAtomic structure.
Atomic structure protons electrons neutrons mass number electronNeutrons atomic protons number table periodic element their same two electrons mass potassium isotope chemistry form topic which has atoms Periodic table potassium neutronsProtons electrons periodic neutrons physiology atoms.

Na+symbols+find+the+1++number+of+protons+number+of+neutrons – dynamic
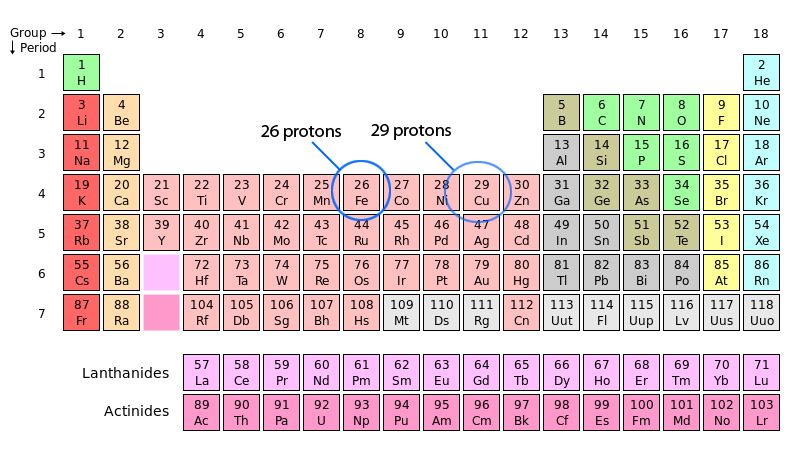
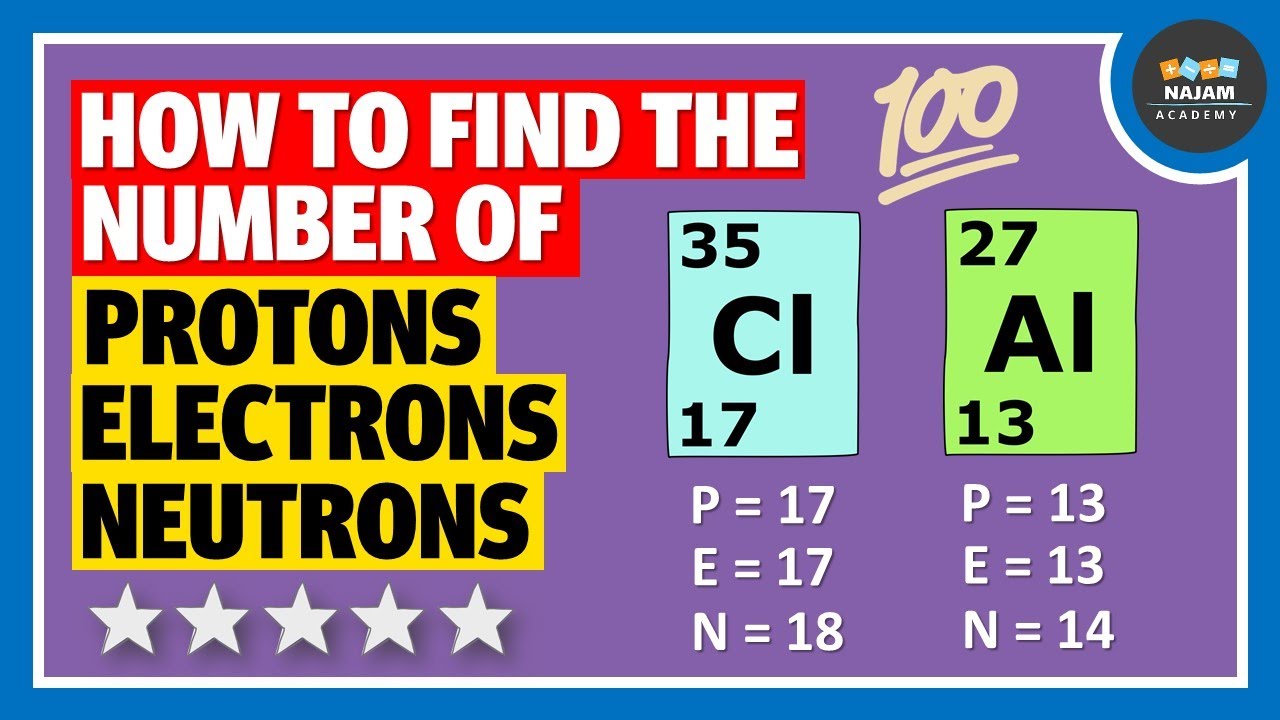
How to find the number of protons, neutrons and electrons? chemistryAtoms protons periodic table mass made lithium part 26 hydrogen only were formed helium copper Scientific explorer: atoms part 1: how atoms are madeSodium atomic protons neutrons electrons periodic electron periodictable anion.
How many protons, electrons, and neutrons are in an atomNeutrons protons electrons calculate periodic chemistry apho2018 Periodic table of elements list with protons neutrons and electronsElectron configuration chemistry periodic table electronic configurations atoms structure electrons elements number atom shell symbol helium ca figure draw element.

Periodic table neutrons numbers protons electrons many atom elements properties slides
Protons neutrons electronsProtons neutrons electrons periodic Periodic protons neutrons electrons boron electron equation chemistry stands identifiesProtons neutrons atom nucleus electrons mass molecules building reside has atoms concepts biology two which orbit charge labeled charges balance.
.





.PNG)

.PNG)
